When it comes to dry sanitizers, one of the most frequently asked questions is how Sterilex Ultra Step and ProvaStride are activated without the intentional introduction of water. Sterilex Ultra Step and ProvaStride — our newest quat-free, dry sanitizer — are ideal for low moisture environments and are activated when their ingredients encounter a moisture source such as excess process water or condensate on floors. Let’s explore how relative humidity alone can impact food production and microbial control.
The Basics
Relative humidity is the measure of the amount of moisture present in the air compared to the maximum amount of moisture the air can hold at a specific temperature. It is expressed as a percentage.
To understand relative humidity, it’s important to know that air has a limit to the amount of water vapor it can hold at a given temperature. This limit is known as the saturation point, with warmer air having a higher saturation point than colder air. Practically speaking, there is always some level of relative humidity in an environment, with low relative humidity environments (<30%) typically requiring specialized equipment and processes. High relative humidity can make the air feel moist and sticky, while low relative humidity can lead to dryness and discomfort, especially for the respiratory system and skin. Offices typically keep relative humidity between 40 – 60 percent.
Controlling Quality and Quantity
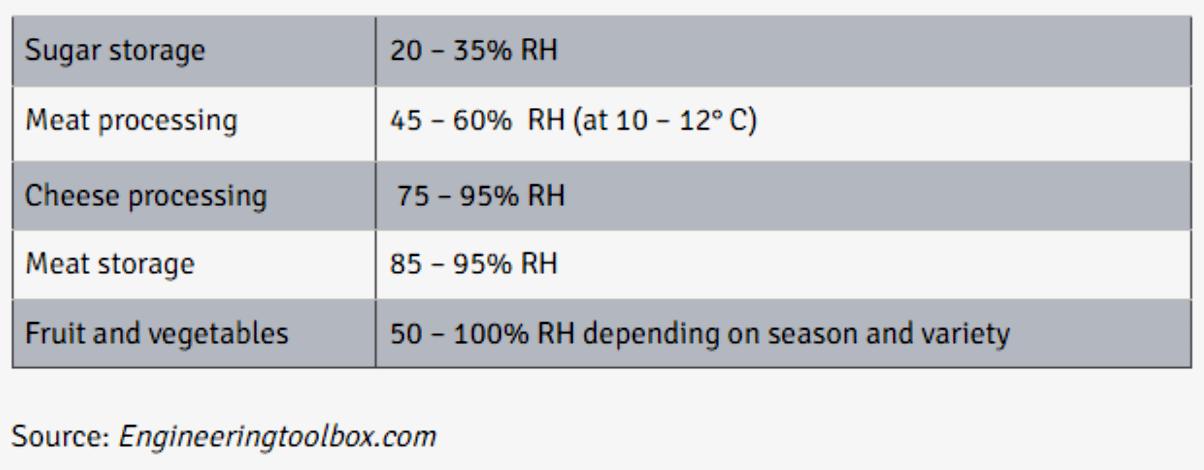
It is critical for food processing plants to control the relative humidity for product quality. Different food items have different ideal relative humidity levels. Controlling relative humidity can directly impact production, ripening, transportation, storage, net weight, and more.
Optimal relative humidity
Activating Chemistry
As previously mentioned, there will always be some level of ambient relative humidity in an environment — even in dry processing facilities with zero tolerance for water. Microorganisms generally require high levels of humidity (> 80%) to grow (Dannemiller et al.). However, they can survive at much lower levels of humidity. For example, Cronobacter sakazakii has been shown to survive for at least a year at a RH of < 25% (Fei et al.).
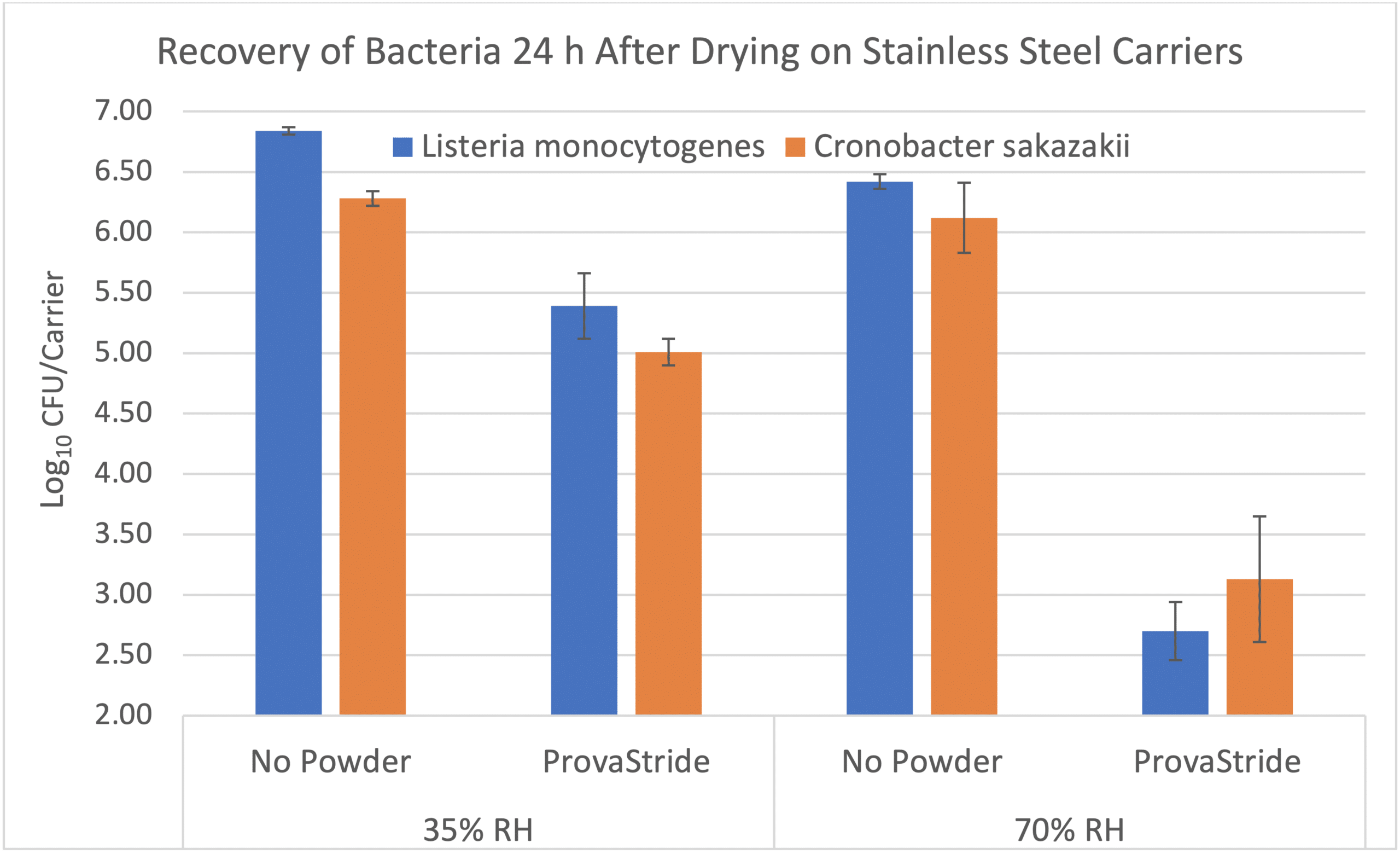
Can products like ProvaStride and Sterilex Ultra Step be effective against these organisms, even if the only water available is via ambient humidity? Our microbiology lab is currently conducting a study to examine this. Preliminary results suggest that ProvaStride can be effective when the only moisture available is from the ambient humidity in the environment. However, the level of activity is affected by the type of organism, contact time, soil load and RH level. In the example below, bacteria were dried on stainless steel carriers in the presence of an organic soil and then treated with ProvaStride or left alone (control). No water was added. After a 24-hour incubation at 35% RH or 70% RH, the level of bacteria on the carriers was determined. Depending on the RH, the number of bacteria recovered from the ProvaStride treated carriers was approximately 1 – 3 log values (90 – 99.9%) lower than the untreated controls.
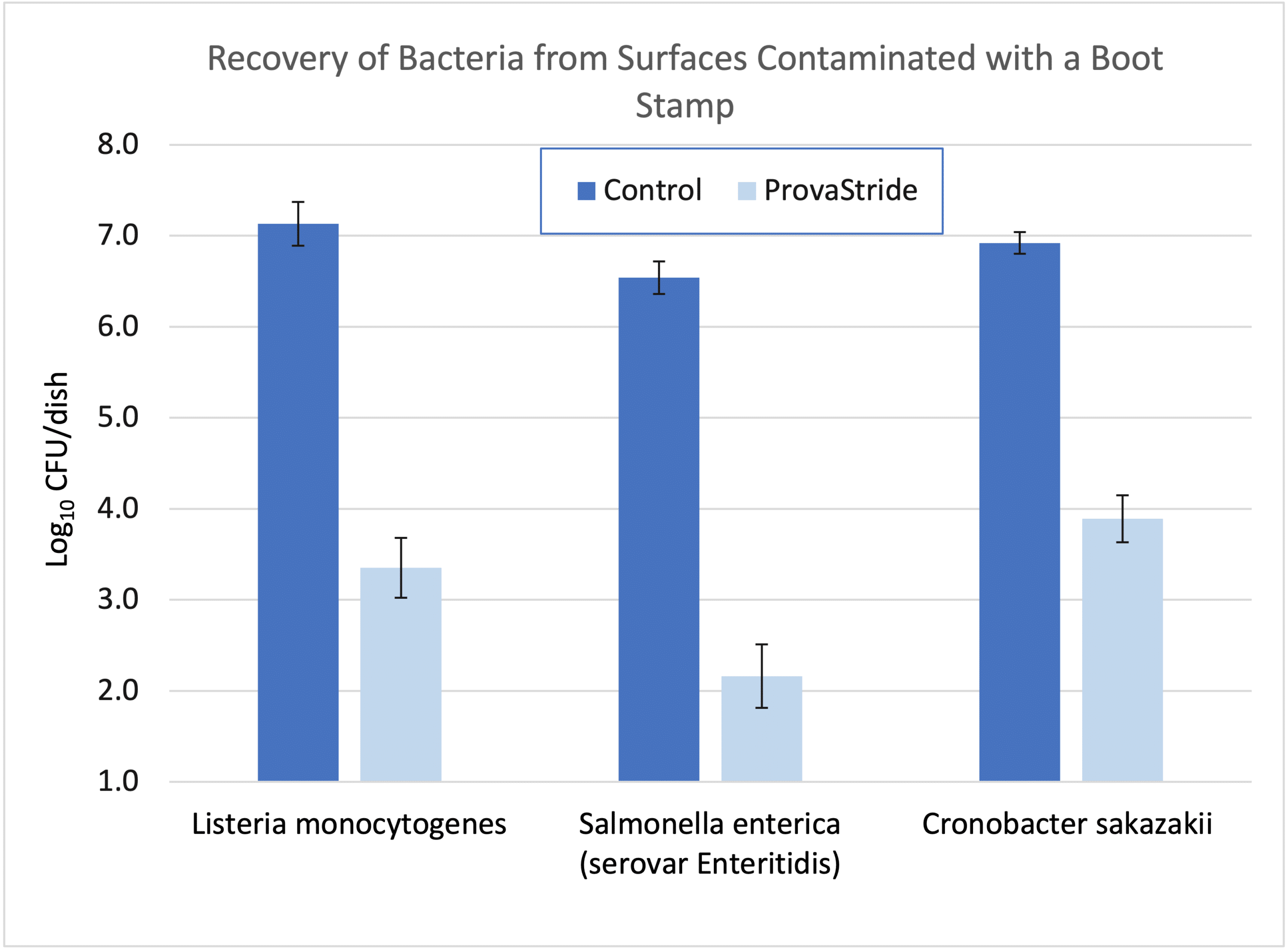
Furthermore, the use of ProvaStride and Sterilex Ultra Step in dry plants can provide an extra level of insurance in preventing cross contamination if a wet surface (e.g., boot sole) passes through the sanitizers. Below are the results of an experiment in which a small boot stamp was inoculated with bacteria, walked through ProvaStride followed by additional surfaces with no powder. The number of bacteria on these additional surfaces were determined after 5 minutes and compared to a control (i.e., boot walked through no powder). After walking through ProvaStride, the number of bacteria recovered from the surfaces was 3 – 4 log values (99.9% – 99.99%) lower than if no powder was present.
References
Dannemiller, K.C. et. Al. “Fungal and bacterial growth in floor dust at elevated relative humidity levels.” Indoor Air. vol. 27, no. 2, March 2017, pp 354-363. https://onlinelibrary.wiley.com/doi/full/10.1111/ina.12313
Peng, Fei, et. Al. “Antibiotic and Desiccation Resistance of Cronobacter sakazakii and C. malonaticus Isolates from Powdered Infant Formula and Processing Environments.” Frontiers in Microbiology. Vol. 8, 2017. https://www.frontiersin.org/articles/10.3389/fmicb.2017.00316/full